Chapter 12 the Lymphatic System and Body Defenses Review Questions Answers
Lymphocytes are responsible for the astonishing specificity of adaptive immune responses. They occur in large numbers in the blood and lymph (the colorless fluid in the lymphatic vessels that connect the lymph nodes in the body to each other and to the bloodstream) and in lymphoid organs, such equally the thymus, lymph nodes, spleen, and appendix (Figure 24-iii).
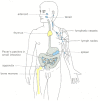
Figure 24-3
Human lymphoid organs. Lymphocytes develop in the thymus and bone marrow (yellow), which are therefore called primal (or primary) lymphoid organs. The newly formed lymphocytes migrate from these main organs to peripheral (or secondary) lymphoid organs (more than...)
In this section, we discuss the full general properties of lymphocytes that apply to both B cells and T cells. Nosotros shall see that each lymphocyte is committed to respond to a specific antigen and that its response during its showtime encounter with an antigen ensures that a more rapid and effective response occurs on subsequent encounters with the same antigen. We consider how lymphocytes avoid responding to self antigens and how they continuously recirculate between the blood and lymphoid organs, ensuring that a lymphocyte will detect its specific foreign antigen no matter where the anitgen enters the body.
Lymphocytes Are Required for Adaptive Immunity
In that location are virtually 2 × 1012 lymphocytes in the man body, making the immune system comparable in cell mass to the liver or encephalon. Despite their abundance, their central function in adaptive immunity was non demonstrated until the belatedly 1950s. The crucial experiments were performed in mice and rats that were heavily irradiated to kill most of their white claret cells, including lymphocytes. This treatment makes the animals unable to mountain adaptive immune responses. Then, by transferring diverse types of cells into the animals it was possible to make up one's mind which cells reversed the deficiency. But lymphocytes restored the adaptive allowed responses of irradiated animals, indicating that lymphocytes are required for these responses (Figure 24-4).

Figure 24-4
A classic experiment showing that lymphocytes are required for adaptive immune responses to strange antigens. An important requirement of all such cell-transfer experiments is that cells are transferred between animals of the same inbred strain. Members (more...)
The Innate and Adaptive Immune Systems Piece of work Together
Equally mentioned earlier, lymphocytes usually respond to foreign antigens only if the innate immune organisation is showtime activated. Every bit discussed in Chapter 25, the innate allowed responses to an infection are rapid. They depend on blueprint recognition receptors that recognize patterns of pathogen-associated molecules (immunostimulants) that are non present in the host organism, including microbial Dna, lipids, and polysaccharides, and proteins that form bacterial flagella. Some of these receptors are present on the surface of professional phagocytic cells such as macrophages and neutrophils, where they mediate the uptake of pathogens, which are and so delivered to lysosomes for devastation. Others are secreted and bind to the surface of pathogens, marking them for destruction by either phagocytes or the complement system. Still others are present on the surface of diverse types of host cells and activate intracellular signaling pathways in response to the bounden of pathogen-associated immunostimulants; this leads to the product of extracellular signal molecules that promote inflammation and help activate adaptive immune responses.
Some cells of the innate immune organisation straight present microbial antigens to T cells to initiate an adaptive immune response. The cells that do this most efficiently are called dendritic cells, which are present in about vertebrate tissues. They recognize and phagocytose invading microbes or their products at a site of infection and and so migrate with their prey to a nearby peripheral lymphoid organ. At that place they act as antigen-presenting cells, which directly activate T cells to respond to the microbial antigens. Once activated, some of the T cells and then migrate to the site of infection, where they assistance other phagocytic cells, mainly macrophages, destroy the microbes (Figure 24-v). Other activated T cells remain in the lymphoid organ and assist B cells reply to the microbial antigens. The activated B cells secrete antibodies that circulate in the body and coat the microbes, targeting them for efficient phagocytosis.
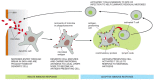
Figure 24-5
One way in which the innate immune system helps activate the adaptive allowed organisation. Specialized phagocytic cells of the innate immune organization, including macrophages (non shown) and dendritic cells ingest invading microbes or their products at the site (more...)
Thus, innate allowed responses are activated mainly at sites of infection, whereas adaptive immune responses are activated in peripheral lymphoid organs. The two types of responses work together to eliminate invading pathogens.
B Lymphocytes Develop in the Bone Marrow; T Lymphocytes Develop in the Thymus
T cells and B cells derive their names from the organs in which they develop. T cells develop in the thymus, and B cells, in mammals, develop in the bone marrow in adults or the liver in fetuses.
Despite their dissimilar origins, both T and B cells develop from the aforementioned pluripotent hemopoietic stem cells, which give ascension to all of the blood cells, including crimson blood cells, white blood cells, and platelets. These stalk cells (discussed in Chapter 22) are located primarily in hemopoietic tissues—mainly the liver in fetuses and the bone marrow in adults. T cells develop in the thymus from precursor cells that drift at that place from the hemopoietic tissues via the blood. In most mammals, including humans and mice, B cells develop from stem cells in the hemopoietic tissues themselves (Figure 24-half-dozen). Because they are sites where lymphocytes develop from forerunner cells, the thymus and hemopoietic tissues are referred to as fundamental (master) lymphoid organs (see Effigy 24-3).
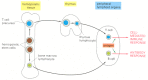
Effigy 24-six
The development and activation of T and B cells. The central lymphoid organs, where lymphocytes develop from precursor cells, are labeled in yellowish boxes. Lymphocytes answer to antigen in peripheral lymphoid organs, such as lymph nodes or spleen.
As nosotros discuss afterwards, most lymphocytes die in the cardinal lymphoid organ presently after they develop, without ever functioning. Others, even so, mature and drift via the claret to the peripheral (secondary) lymphoid organs—mainly, the lymph nodes, spleen, and epithelium-associated lymphoid tissues in the alimentary canal, respiratory tract, and skin (run across Figure 24-3). As mentioned earlier, it is in the peripheral lymphoid organs that T cells and B cells react with strange antigens (see Figure 24-6).
T and B cells become morphologically distinguishable from each other but later on they have been activated by antigen. Nonactivated T and B cells look very similar, even in an electron microscope. Both are small, only marginally bigger than red blood cells, and contain fiddling cytoplasm (Figure 24-7A). Both are activated by antigen to proliferate and mature into effector cells. Effector B cells secrete antibodies. In their most mature form, called plasma cells, they are filled with an extensive rough endoplasmic reticulum (Figure 24-7B). In dissimilarity, effector T cells (Effigy 24-7C) comprise very trivial endoplasmic reticulum and exercise not secrete antibodies.

Effigy 24-7
Electron micrographs of nonactivated and activated lymphocytes. (A) A resting lymphocyte, which could be a T cell or a B jail cell, every bit these cells are difficult to distinguish morphologically until they have been activated to become effector cells. (B) An (more...)
There are two primary classes of T cells—cytotoxic T cells and helper T cells. Cytotoxic T cells impale infected cells, whereas helper T cells aid activate macrophages, B cells, and cytotoxic T cells. Effector helper T cells secrete a variety of signal proteins called cytokines, which act equally local mediators. They also brandish a multifariousness of costimulatory proteins on their surface. By means of these cytokines and membrane-bound costimulatory proteins, they can influence the behavior of the various cell types they help. Effector cytotoxic T cells kill infected target cells also past means of proteins that they either secrete or display on their surface. Thus, whereas B cells can deed over long distances by secreting antibodies that are distributed by the bloodstream, T cells can drift to distant sites, but in that location they act simply locally on neighboring cells.
The Adaptive Immune System Works by Clonal Selection
The nearly remarkable feature of the adaptive allowed system is that it can respond to millions of different foreign antigens in a highly specific way. B cells, for example, brand antibodies that react specifically with the antigen that induced their production. How practise B cells produce such a diversity of specific antibodies? The answer began to sally in the 1950s with the formulation of the clonal selection theory. According to this theory, an animal first randomly generates a vast diversity of lymphocytes, then those lymphocytes that can react against the strange antigens that the brute actually encounters are specifically selected for action. As each lymphocyte develops in a key lymphoid organ, it becomes committed to react with a particular antigen before ever existence exposed to the antigen. It expresses this delivery in the form of jail cell-surface receptor proteins that specifically fit the antigen. When a lymphocyte encounters its antigen in a peripheral lymphoid organ, the binding of the antigen to the receptors activates the lymphocyte, causing it both to proliferate and to differentiate into an effector jail cell. An antigen therefore selectively stimulates those cells that express complementary antigen-specific receptors and are thus already committed to respond to information technology. This arrangement is what makes adaptive immune responses antigen-specific.
The term "clonal" in clonal selection theory derives from the postulate that the adaptive immune system is composed of millions of different families, or clones, of lymphocytes, each consisting of T or B cells descended from a common ancestor. Each ancestral prison cell was already committed to brand ane particular antigen-specific receptor protein, and so all cells in a clone accept the same antigen specificity (Figure 24-eight). According to the clonal selection theory, so, the immune organization functions on the "ready-made" principle rather than the "made-to-order" i.

Figure 24-8
The clonal option theory. An antigen activates only those lymphocyte clones (represented here by unmarried cells) that are already committed to reply to information technology. A cell committed to respond to a particular antigen displays cell-surface receptors that specifically (more than...)
There is compelling evidence to support the main tenets of the clonal pick theory. For example, when lymphocytes from an creature that has non been immunized are incubated in a test tube with a number of radioactively labeled antigens, only a very small proportion (less than 0.01%) bind each antigen, suggesting that just a few cells are committed to respond to these antigens. Moreover, when one antigen is made so highly radioactive that it kills any cell that it binds to, the remaining lymphocytes can no longer produce an immune response to that particular antigen, even though they can still reply commonly to other antigens. Thus, the committed lymphocytes must have receptors on their surface that specifically bind that antigen. Although most experiments of this kind have involved B cells and antibody responses, other experiments indicate that T cells, similar B cells, operate by clonal choice.
How can the adaptive immune arrangement produce lymphocytes that collectively display such an enormous diversity of receptors, including ones that recognize synthetic molecules that never occur in nature? Nosotros shall come across later that the antigen-specific receptors on both T and B cells are encoded by genes that are assembled from a series of gene segments by a unique grade of genetic recombination that occurs early in a lymphocyte'south development, earlier it has encountered antigen. This associates procedure generates the enormous diversity of receptors and lymphocytes, thereby enabling the immune arrangement to respond to an well-nigh unlimited multifariousness of antigens.
Well-nigh Antigens Activate Many Different Lymphocyte Clones
Most large molecules, including virtually all proteins and many polysaccharides, tin can serve as antigens. Those parts of an antigen that combine with the antigen-binding site on either an antibody molecule or a lymphocyte receptor are called antigenic determinants (or epitopes). Almost antigens have a variety of antigenic determinants that can stimulate the production of antibodies, specific T jail cell responses, or both. Some determinants of an antigen produce a greater response than others, and so that the reaction to them may dominate the overall response. Such determinants are said to be immunodominant.
The diversity of lymphocytes is such that fifty-fifty a single antigenic determinant is likely to activate many clones, each of which produces an antigen-binding site with its own feature affinity for the determinant. Even a relatively uncomplicated structure, like the dinitrophenyl (DNP) grouping in Figure 24-9, tin be "looked at" in many ways. When information technology is coupled to a protein, every bit shown in the figure, information technology commonly stimulates the production of hundreds of species of anti-DNP antibodies, each made by a different B cell clone. Such responses are said to be polyclonal. When only a few clones are activated, the response is said to be oligoclonal; and when the response involves but a unmarried B or T cell clone, information technology is said to be monoclonal. Monoclonal antibodies are widely used equally tools in biology and medicine, but they take to be produced in a special way (see Figure eight-half dozen), as the responses to virtually antigens are polyclonal.
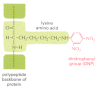
Figure 24-9
The dinitrophenyl (DNP) group. Although it is too modest to induce an immune response on its own, when it is coupled covalently to a lysine side concatenation on a protein, every bit illustrated, DNP stimulates the product of hundreds of different species of antibodies (more than...)
Immunological Retentiveness Is Due to Both Clonal Expansion and Lymphocyte Differentiation
The adaptive immune system, similar the nervous system, can call up prior experiences. This is why we develop lifelong immunity to many common infectious diseases afterwards our initial exposure to the pathogen, and it is why vaccination works. The same phenomenon can be demonstrated in experimental animals. If an animal is immunized once with antigen A, an immune response (either antibody or jail cell-mediated) appears later several days, rises rapidly and exponentially, and then, more than gradually, declines. This is the characteristic course of a primary allowed response, occurring on an beast's offset exposure to an antigen. If, afterwards some weeks, months, or even years have elapsed, the animal is reinjected with antigen A, it will usually produce a secondary immune response that is very different from the primary response: the lag period is shorter, and the response is greater. These differences indicate that the creature has "remembered" its starting time exposure to antigen A. If the animal is given a different antigen (for example, antigen B) instead of a second injection of antigen A, the response is typical of a principal, and non a secondary, allowed response. The secondary response must therefore reflect antigen-specific immunological retentivity for antigen A (Figure 24-10).
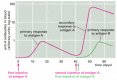
Effigy 24-10
Primary and secondary antibiotic responses. The secondary response induced by a second exposure to antigen A is faster and greater than the primary response and is specific for A, indicating that the adaptive immune arrangement has specifically remembered encountering (more...)
The clonal selection theory provides a useful conceptual framework for understanding the cellular basis of immunological retentivity. In an adult animal, the peripheral lymphoid organs incorporate a mixture of cells in at least three stages of maturation: naïve cells, effector cells and retentivity cells. When naïve cells encounter antigen for the beginning time, some of them are stimulated to proliferate and differentiate into effector cells, which are actively engaged in making a response (effector B cells secrete antibody, while effector T cells impale infected cells or assistance other cells fight the infection). Instead of becoming effector cells, some naïve cells are stimulated to multiply and differentiate into memory cells—cells that are not themselves engaged in a response simply are more easily and more quickly induced to become effector cells by a later encounter with the same antigen. Retentiveness cells, similar naïve cells, requite ascent to either effector cells or more retentivity cells (Figure 24-eleven).
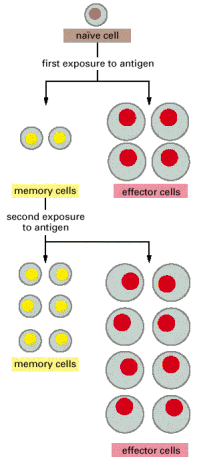
Figure 24-11
A model for the cellular footing of immunological retention. When naïve lymphocytes are stimulated by their specific antigen, they proliferate and differentiate. Most get effector cells which role so die, while others go long-lived (more...)
Thus, immunological memory is generated during the chief response in part because the proliferation of antigen-stimulated naïve cells creates many memory cells—a procedure known as clonal expansion—and in part because memory cells are able to respond more than sensitively and rapidly to the same antigen than practise naïve cells. And, unlike most effector cells, which die within days or weeks, memory cells can live for the lifetime of the animate being, thereby providing lifelong immunological memory.
Acquired Immunological Tolerance Ensures That Self Antigens Are Not Attacked
Equally discussed in Chapter 25, cells of the innate immune system recognize molecules on the surface of pathogens that are not constitute in the host. The adaptive immune organisation has a far more difficult recognition task: it must be able to respond specifically to an almost unlimited number of strange macromolecules, while avoiding responding to the large number of molecules made past the host organism itself. How does it exercise information technology? For one affair, self molecules practise not induce the innate immune reactions that are required to actuate adaptive immune responses. But fifty-fifty when an infection triggers innate reactions, self molecules nevertheless practise not usually induce adaptive immune responses. Why non?
One answer is that the adaptive immune organization "learns" not to reply to cocky antigens. Transplantation experiments provide one line of evidence for this learning process. When tissues are transplanted from one private to another, as long as the two individuals are not identical twins, the immune organization of the recipient usually recognizes the donor cells as foreign and destroys them. (For reasons we hash out afterwards, the foreign antigens on the donor cells are and so powerful that they tin can stimulate adaptive allowed responses in the absence of infection or an adjuvant.) If, even so, cells from ane strain of mouse are introduced into a neonatal mouse of some other strain, some of these cells survive for most of the recipient animal'southward life, and the recipient will now accept a graft from the original donor, even though information technology rejects "third-party" grafts. Plain, nonself antigens can, in some circumstances, induce the allowed system to go specifically unresponsive to them. This antigen-specific unresponsiveness to foreign antigens is known as acquired immunological tolerance (Figure 24-12).
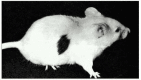
Figure 24-12
Immunological tolerance. The skin graft seen here, transplanted from an adult brownish mouse to an adult white mouse, has survived for many weeks only considering the white mouse, at the time of its nascence, received an injection of cells from the brown mouse (more than...)
The unresponsiveness of an animal's adaptive allowed organisation to its own macromolecules (natural immunological tolerance) is acquired in the aforementioned style. Normal mice, for instance, cannot brand an immune response against one of their own protein components of the complement system called C5 (discussed in Chapter 25). Mutant mice, however, that lack the factor encoding C5 (but are otherwise genetically identical to the normal mice) can brand a strong allowed response to this blood protein when immunized with it. Natural immunological tolerance for a item self molecule persists just for as long as the molecule remains nowadays in the body. If a self molecule such equally C5 is removed, an animal gains the power to respond to it after a few weeks or months. Thus, the allowed system is genetically capable of responding to self molecules but learns not to do so.
The learning procedure that leads to self-tolerance can involve killing the self-reactive lymphocytes (clonal deletion), functionally inactivating them (clonal anergy or inactivation), stimulating the cells to produce modified receptors that no longer recognize the self antigen (receptor editing), or the suppression of self-reactive lymphocytes past a special type of regulatory T cell. The procedure begins in the central lymphoid organs when newly formed self-reactive lymphocytes showtime encounter their self antigen. Instead of being activated by binding antigen, the young lymphocytes are induced to either alter their receptors or dice by apoptosis. Lymphocytes that could potentially reply to self antigens that are not present in the central lymphoid organs oftentimes dice or are either inactivated or suppressed after they take matured and migrated to peripheral lymphoid organs.
Why does the binding of self antigen lead to tolerance rather than activation? Every bit nosotros hash out afterwards, for a lymphocyte to be activated in a peripheral lymphoid organ, it must non only bind its antigen simply must also receive a costimulatory bespeak. The latter bespeak is provided by a helper T jail cell in the case of a B lymphocyte and by an antigen-presenting cell in the case of a T lymphocyte. The production of costimulatory signals commonly depends on exposure to pathogens, and and then a self-reactive lymphocyte normally encounters its antigen in the absence of such signals. Without a costimulatory signal, an antigen tends to impale or inactivate a lymphocyte rather than activate it (Figure 24-13).
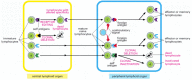
Figure 24-13
Consecration of immunological tolerance to self antigens in primal and peripheral lymphoid organs. When a cocky-reactive young lymphocyte binds its self antigen in the key lymphoid organ where the cell is produced, information technology may be induced to modify the receptor (more...)
Tolerance to self antigens sometimes breaks downward, causing T or B cells (or both) to react against the organism's own tissue antigens. Myasthenia gravis is an example of such an autoimmune disease. Affected individuals make antibodies against the acetylcholine receptors on their own skeletal muscle cells. These antibodies interfere with the normal performance of the receptors and then that the patients get weak and may dice considering they cannot breathe. The mechanisms responsible for the breakdown of tolerance to cocky antigens in autoimmune diseases are unknown. It is idea, however, that activation of the innate immune arrangement by infection may help trigger certain anti-self responses in genetically susceptible individuals.
Lymphocytes Continuously Circulate Through Peripheral Lymphoid Organs
Pathogens by and large enter the body through an epithelial surface, usually through the skin, gut, or respiratory tract. How do the microbial antigens travel from these entry points to a peripheral lymphoid organ, such as a lymph node or the spleen, where lymphocytes are activated (see Effigy 24-six)? The route and destination depend on the site of entry. Antigens that enter through the peel or respiratory tract are carried via the lymph to local lymph nodes; those that enter through the gut end up in gut-associated peripheral lymphoid organs such as Peyer's patches; and those that enter the blood are filtered out in the spleen. In well-nigh cases, dendritic cells acquit the antigen from the site of infection to the peripheral lymphoid organ, where they become antigen-presenting cells (see Figure 24-5), specialized for activating T cells (as we discuss later).
But the lymphocytes that tin can recognize a particular microbial antigen in a peripheral lymph organ are only a tiny fraction of the full lymphocyte population. How practise these rare cells find an antigen-presenting cell displaying their antigen? The answer is that they continuously circulate between the lymph and claret until they meet their antigen. In a lymph node, for example, lymphocytes continually leave the bloodstream by squeezing out between specialized endothelial cells lining pocket-size veins chosen postcapillary venules. Later on percolating through the node, they accumulate in minor lymphatic vessels that leave the node and connect with other lymphatic vessels that pass through other lymph nodes downstream (see Figure 24-three). Passing into larger and larger vessels, the lymphocytes eventually enter the chief lymphatic vessel (the thoracic duct), which carries them dorsum into the blood (Effigy 24-fourteen). This continuous recirculation between the claret and lymph ends simply if a lymphocyte encounters its specific antigen (and a costimulatory signal) on the surface of an antigen-presenting cell in a peripheral lymphoid organ. Now the lymphocyte is retained in the peripheral lymphoid organ, where it proliferates and differentiates into effector cells. Some of the effector T cells and then go out the organ via the lymph and drift through the blood to the site of infection (see Figure 24-5).
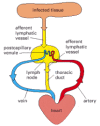
Figure 24-14
The path followed past lymphocytes as they continuously broadcast betwixt the lymph and claret. The circulation through a lymph node is shown hither. Microbial antigens are carried into the lymph node by dendritic cells, which enter via afferent lymphatic (more than...)
Lymphocyte recirculation depends on specific interactions between the lymphocyte cell surface and the surface of the specialized endothelial cells lining the postcapillary venules in the peripheral lymphoid organs. Many prison cell types in the blood come into contact with these endothelial cells, only only lymphocytes adhere so migrate out of the bloodstream. The lymphocytes initially adhere to the endothelial cells via homing receptors that bind to specific ligands (oft chosen counterreceptors) on the endothelial prison cell surface. Lymphocyte migration into lymph nodes, for case, depends on a homing receptor protein called L-selectin, a member of the selectin family of cell-surface lectins discussed in Chapter xix. This protein binds to specific carbohydrate groups on a counterreceptor that is expressed exclusively on the surface of the specialized endothelial cells in lymph nodes, causing the lymphocytes to adhere weakly to the endothelial cells and to gyre slowly along their surface. The rolling continues until another, much stronger adhesion arrangement is called into play by chemo-attractant proteins (called chemokines; see below) secreted past endothelial cells. This strong adhesion is mediated past members of the integrin family unit of jail cell adhesion molecules (discussed in Chapter xix), which become activated on the lymphocyte surface. Now the lymphocytes cease rolling and crawl out of the blood vessel into the lymph node (Figure 24-15).
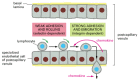
Figure 24-15
Migration of a lymphocyte out of the bloodstream into a lymph node. A circulating lymphocyte adheres weakly to the surface of the specialized endothelial cells lining a postcapillary venule in a lymph node. This initial adhesion is mediated by L-selectin (more...)
Chemokines are small, secreted, positively charged proteins that have a central role in guiding the migrations of various types of white blood cells. They are all structurally related and bind to the surface of endothelial cells, and to negatively charged proteoglycans of the extracellular matrix in organs. Past bounden to Yard-protein-linked receptors (discussed in Chapter 15) on the surface of specific blood cells, chemokines attract these cells from the bloodstream into an organ, guide them to specific locations within the organ, and and so help cease migration. (The AIDS virus (HIV) also binds to chemokine receptors, which allows the virus to infect white blood cells.) T and B cells initially enter the same region of a lymph node simply are and then attracted by different chemokines to separate regions of the node—T cells to the paracortex and B cells to lymphoid follicles (Figure 24-16). Unless they run into their antigen, both types of cells soon leave the lymph node via lymphatic vessels. If they meet their antigen, all the same, they remain in the node, proliferate, and differentiate into either effector cells or memory cells. Most of the effector cells go out the node, expressing different chemokine receptors that help guide them to their new destinations—T cells to sites of infection and B cells to the os marrow.
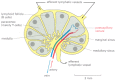
Figure 24-16
A simplified drawing of a human lymph node. B cells are primarily amassed in structures chosen lymphoid follicles, whereas T cells are institute mainly in the paracortex. Both types of lymphocytes are attracted past chemokines to enter the lymph node from (more...)
Summary
Innate immune responses are triggered at sites of infection by microbe-specific molecules associated with invading pathogens. In add-on to fighting infection direct, these responses help activate adaptive immune responses in peripheral lymphoid organs. Unlike innate immune responses, adaptive responses provide specific and long-lasting protection against the particular pathogen that induced them.
The adaptive allowed system is composed of millions of lymphocyte clones, with the cells in each clone sharing a unique cell-surface receptor that enables them to bind a particular antigen. The bounden of antigen to these receptors, however, is usually not sufficient to stimulate a lymphocyte to proliferate and differentiate into an effector cell that tin can assist eliminate the pathogen. Costimulatory signals provided by some other specialized cell in a peripheral lymphoid organ are likewise required. Helper T cells provide such signals for B cells, while antigen-presenting dendritic cells commonly provide them for T cells. Effector B cells secrete antibodies, which can act over long distances to aid eliminate extracellular pathogens and their toxins. Effector T cells, by contrast, act locally at sites of infection to either kill infected host cells or help other cells to eliminate pathogens. Equally part of the adaptive allowed response, some lymphocytes proliferate and differentiate into retentivity cells, which are able to respond faster and more efficiently the next time the same pathogen invades. Lymphocytes that would react against cocky molecules are either induced to alter their receptors, induced to kill themselves, inactivated, or suppressed, and so that the adaptive immune system normally reacts only against foreign antigens. Both B and T cells circulate continuously betwixt the claret and lymph. Just if they encounter their specific foreign antigen in a peripheral lymphoid organ do they cease migrating, proliferate, and differentiate into effector cells or memory cells.

Source: https://www.ncbi.nlm.nih.gov/books/NBK26921/
0 Response to "Chapter 12 the Lymphatic System and Body Defenses Review Questions Answers"
Post a Comment